Running simulations#
In this tutorial, we are going to cover the following topics:
How to merge compartments into compartmental neuron models
How to make Dendrify and Brian interact with each other
How to run simulations of Dendrify models in Brian
Disclaimer: Below, we present a generic "toy" model developed solely for educational purposes. However, please note that its parameters and behavior have not been validated using real data. If you intend to utilize Dendrify in a project, we strongly advise against using this model as it is, unless you first calibrate its parameters to align with your specific requirements.
Imports & settings#
import brian2 as b
import matplotlib.pyplot as plt
from brian2.units import *
from dendrify import Soma, Dendrite, NeuronModel
b.prefs.codegen.target = 'numpy' # faster for basic models and short simulations
# Plot settings
blue = '#005c94ff'
green = '#338000ff'
orange = '#ff6600ff'
notred = '#aa0044ff'
params = {
"legend.fontsize": 10,
"legend.handlelength": 1.5,
"legend.edgecolor": 'inherit',
"legend.columnspacing": 0.8,
"legend.handletextpad": 0.5,
"axes.labelsize": 10,
"axes.titlesize": 11,
"axes.spines.right": False,
"axes.spines.top": False,
"xtick.labelsize": 10,
"ytick.labelsize": 10,
'lines.markersize': 3,
'lines.linewidth': 1.25,
'grid.color': "#d3d3d3",
'figure.dpi': 150,
'axes.prop_cycle': b.cycler(color=[blue, green, orange, notred])
}
plt.rcParams.update(params)
Create a compartmental model#
Lets try to recreate the following basic 4-compartment model:
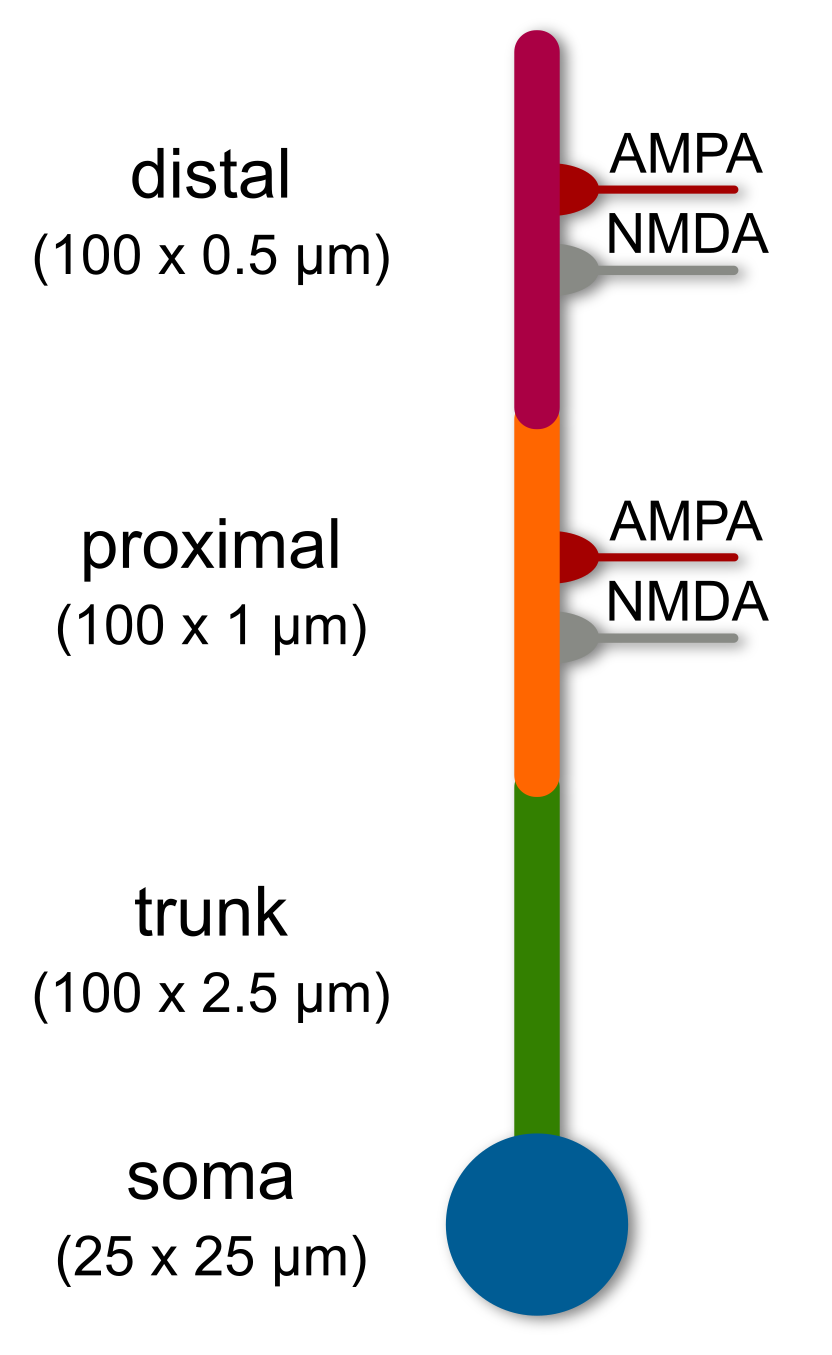
According to the previous tutorial the code should look somethink like this:
# create soma
soma = Soma('soma', model='leakyIF', length=25*um, diameter=25*um,
cm=1*uF/(cm**2), gl=40*uS/(cm**2),
v_rest=-65*mV)
# create trunk
trunk = Dendrite('trunk', length=100*um, diameter=2.5*um,
cm=1*uF/(cm**2), gl=40*uS/(cm**2),
v_rest=-65*mV)
# create proximal dendrite
prox = Dendrite('prox', length=100*um, diameter=1*um,
cm=1*uF/(cm**2), gl=40*uS/(cm**2),
v_rest=-65*mV)
prox.synapse('AMPA', tag='pathY', g=1*nS, t_decay=2*ms)
prox.synapse('NMDA', tag='pathY', g=1*nS, t_decay=60*ms)
# create distal dendrite
dist = Dendrite('dist', length=100*um, diameter=0.5*um,
cm=1*uF/(cm**2), gl=40*uS/(cm**2),
v_rest=-65*mV)
dist.synapse('AMPA', tag='pathX', g=1*nS, t_decay=2*ms)
dist.synapse('NMDA', tag='pathX', g=1*nS, t_decay=60*ms)
soma.connect(trunk, g=15*nS)
trunk.connect(prox, g=6*nS)
prox.connect(dist, g=2*nS)
HOWEVER: There is a far better way for creating a compartmental model!!
# create compartments
soma = Soma('soma', model='leakyIF', length=25*um, diameter=25*um)
trunk = Dendrite('trunk', length=100*um, diameter=2.5*um)
prox = Dendrite('prox', length=100*um, diameter=1*um)
dist = Dendrite('dist', length=100*um, diameter=0.5*um)
# add AMPA/NMDA synapses
prox.synapse('AMPA', tag='pathY', g=1*nS, t_decay=2*ms)
prox.synapse('NMDA', tag='pathY', g=1*nS, t_decay=60*ms)
dist.synapse('AMPA', tag='pathX', g=1*nS, t_decay=2*ms)
dist.synapse('NMDA', tag='pathX', g=1*nS, t_decay=60*ms)
# merge compartments into a neuron model and set its basic properties
graph = [(soma, trunk, 15*nS), (trunk, prox, 6*nS), (prox, dist, 2*nS)]
model = NeuronModel(graph, cm=1*uF/(cm**2), gl=40*uS/(cm**2),
v_rest=-65*mV, scale_factor=2.8, spine_factor=1.5)
The NeuronModel class, not only allows to set model parameters, but also unlocks many cool functions that we are going to explore now.
print(model)
OBJECT
------
<class 'dendrify.neuronmodel.NeuronModel'>
EQUATIONS
---------
dV_soma/dt = (gL_soma * (EL_soma-V_soma) + I_soma) / C_soma :volt
I_soma = I_ext_soma + I_trunk_soma :amp
I_ext_soma :amp
I_trunk_soma = (V_trunk-V_soma) * g_trunk_soma :amp
dV_trunk/dt = (gL_trunk * (EL_trunk-V_trunk) + I_trunk) / C_trunk :volt
I_trunk = I_ext_trunk + I_prox_trunk + I_soma_trunk :amp
I_ext_trunk :amp
I_soma_trunk = (V_soma-V_trunk) * g_soma_trunk :amp
I_prox_trunk = (V_prox-V_trunk) * g_prox_trunk :amp
dV_prox/dt = (gL_prox * (EL_prox-V_prox) + I_prox) / C_prox :volt
I_prox = I_ext_prox + I_dist_prox + I_trunk_prox + I_NMDA_pathY_prox + I_AMPA_pathY_prox :amp
I_ext_prox :amp
I_AMPA_pathY_prox = g_AMPA_pathY_prox * (E_AMPA-V_prox) * s_AMPA_pathY_prox * w_AMPA_pathY_prox :amp
ds_AMPA_pathY_prox/dt = -s_AMPA_pathY_prox / t_AMPA_decay_pathY_prox :1
I_NMDA_pathY_prox = g_NMDA_pathY_prox * (E_NMDA-V_prox) * s_NMDA_pathY_prox / (1 + Mg_con * exp(-Alpha_NMDA*(V_prox/mV+Gamma_NMDA)) / Beta_NMDA) * w_NMDA_pathY_prox :amp
ds_NMDA_pathY_prox/dt = -s_NMDA_pathY_prox/t_NMDA_decay_pathY_prox :1
I_trunk_prox = (V_trunk-V_prox) * g_trunk_prox :amp
I_dist_prox = (V_dist-V_prox) * g_dist_prox :amp
dV_dist/dt = (gL_dist * (EL_dist-V_dist) + I_dist) / C_dist :volt
I_dist = I_ext_dist + I_prox_dist + I_NMDA_pathX_dist + I_AMPA_pathX_dist :amp
I_ext_dist :amp
I_AMPA_pathX_dist = g_AMPA_pathX_dist * (E_AMPA-V_dist) * s_AMPA_pathX_dist * w_AMPA_pathX_dist :amp
ds_AMPA_pathX_dist/dt = -s_AMPA_pathX_dist / t_AMPA_decay_pathX_dist :1
I_NMDA_pathX_dist = g_NMDA_pathX_dist * (E_NMDA-V_dist) * s_NMDA_pathX_dist / (1 + Mg_con * exp(-Alpha_NMDA*(V_dist/mV+Gamma_NMDA)) / Beta_NMDA) * w_NMDA_pathX_dist :amp
ds_NMDA_pathX_dist/dt = -s_NMDA_pathX_dist/t_NMDA_decay_pathX_dist :1
I_prox_dist = (V_prox-V_dist) * g_prox_dist :amp
PARAMETERS
----------
{'Alpha_NMDA': 0.062,
'Beta_NMDA': 3.57,
'C_dist': 6.59734457 * pfarad,
'C_prox': 13.19468915 * pfarad,
'C_soma': 82.46680716 * pfarad,
'C_trunk': 32.98672286 * pfarad,
'EL_dist': -65. * mvolt,
'EL_prox': -65. * mvolt,
'EL_soma': -65. * mvolt,
'EL_trunk': -65. * mvolt,
'E_AMPA': 0. * volt,
'E_Ca': 136. * mvolt,
'E_GABA': -80. * mvolt,
'E_K': -89. * mvolt,
'E_NMDA': 0. * volt,
'E_Na': 70. * mvolt,
'Gamma_NMDA': 0,
'Mg_con': 1.0,
'gL_dist': 263.8937829 * psiemens,
'gL_prox': 0.52778757 * nsiemens,
'gL_soma': 3.29867229 * nsiemens,
'gL_trunk': 1.31946891 * nsiemens,
'g_AMPA_pathX_dist': 1. * nsiemens,
'g_AMPA_pathY_prox': 1. * nsiemens,
'g_NMDA_pathX_dist': 1. * nsiemens,
'g_NMDA_pathY_prox': 1. * nsiemens,
'g_dist_prox': 2. * nsiemens,
'g_prox_dist': 2. * nsiemens,
'g_prox_trunk': 6. * nsiemens,
'g_soma_trunk': 15. * nsiemens,
'g_trunk_prox': 6. * nsiemens,
'g_trunk_soma': 15. * nsiemens,
't_AMPA_decay_pathX_dist': 2. * msecond,
't_AMPA_decay_pathY_prox': 2. * msecond,
't_NMDA_decay_pathX_dist': 60. * msecond,
't_NMDA_decay_pathY_prox': 60. * msecond,
'w_AMPA_pathX_dist': 1.0,
'w_AMPA_pathY_prox': 1.0,
'w_NMDA_pathX_dist': 1.0,
'w_NMDA_pathY_prox': 1.0}
EVENTS
------
[]
EVENT CONDITIONS
----------------
{}
model.as_graph()
model.as_graph(figsize=[4,3], scale_nodes=0.5, fontsize=8, color_soma='black')
Dendrify meets Brian#
neuron, ap_reset = model.make_neurongroup(1, method='euler', threshold='V_soma > -40*mV',
reset='V_soma = 40*mV',
second_reset= 'V_soma=-55*mV',
spike_width = 0.5*ms,
refractory=4*ms)
# Set a monitor to record the voltages of all compartments
voltages = ['V_soma', 'V_trunk', 'V_prox', 'V_dist']
M = b.StateMonitor(neuron, voltages, record=True)
Run simulation and plot results#
net = b.Network(neuron, ap_reset, M) # organize everythink into a network
net.run(10*ms) # no input
neuron.I_ext_soma = 200*pA
net.run(100*ms) # 200 pA injected at the soma for 100 ms
neuron.I_ext_soma = 0*pA
net.run(60*ms) # run another 60 ms without any input
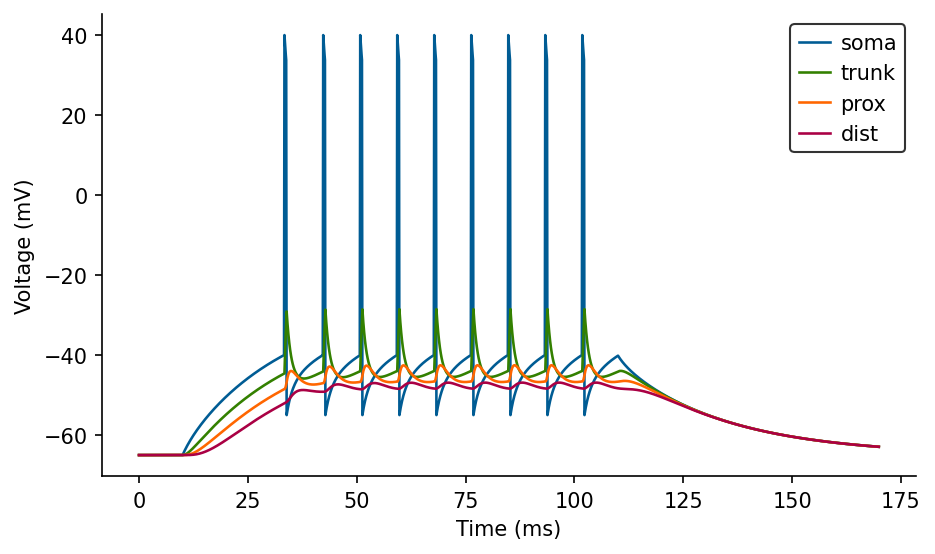
# Plot voltages
fig, ax = plt.subplots(figsize=(7, 4))
ax.plot(M.t/ms, M.V_soma[0]/mV, label='soma')
ax.plot(M.t/ms, M.V_trunk[0]/mV, label='trunk')
ax.plot(M.t/ms, M.V_prox[0]/mV, label='prox')
ax.plot(M.t/ms, M.V_dist[0]/mV, label='dist')
ax.set_xlabel('Time (ms)')
ax.set_ylabel('Voltage (mV)')
ax.legend(loc='best');
# Zoom in
fig, ax = plt.subplots(figsize=(7, 4))
ax.plot(M.t/ms, M.V_soma[0]/mV, label='soma')
ax.plot(M.t/ms, M.V_trunk[0]/mV, label='trunk')
ax.plot(M.t/ms, M.V_prox[0]/mV, label='prox')
ax.plot(M.t/ms, M.V_dist[0]/mV, label='dist')
ax.set_xlabel('Time (ms)')
ax.set_ylabel('Voltage (mV)')
ax.set_xlim(left=40, right=80)
ax.legend(loc='best');
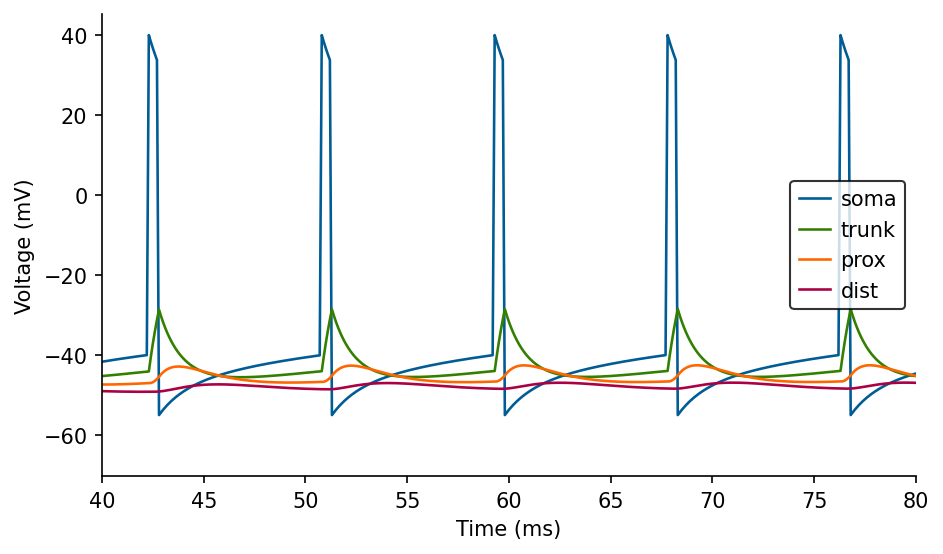
NOTE: The dendritic voltage fluctuations that follow somatic APs are due to the electrical coupling of these compartments. They are not backpropagating dSpikes (not included yet in the model)
A network with random input#
b.start_scope() # clear previous run
# First create 2 input groups
inputX = b.PoissonGroup(50, 10*Hz) # 50 neurons firing at 10 Hz
inputY = b.PoissonGroup(50, 10*Hz) # 50 neurons firing at 10 Hz
# And a NEWronGroup (I am so funny)
group, ap_reset = model.make_neurongroup(100, method='euler', threshold='V_soma > -40*mV',
reset='V_soma = 40*mV',
second_reset= 'V_soma=-55*mV',
spike_width = 0.5*ms,
refractory=4*ms)
# Let's remember how the equations look like:
print(dist.equations)
dV_dist/dt = (gL_dist * (EL_dist-V_dist) + I_dist) / C_dist :volt
I_dist = I_ext_dist + I_NMDA_pathX_dist + I_AMPA_pathX_dist :amp
I_ext_dist :amp
I_AMPA_pathX_dist = g_AMPA_pathX_dist * (E_AMPA-V_dist) * s_AMPA_pathX_dist * w_AMPA_pathX_dist :amp
ds_AMPA_pathX_dist/dt = -s_AMPA_pathX_dist / t_AMPA_decay_pathX_dist :1
I_NMDA_pathX_dist = g_NMDA_pathX_dist * (E_NMDA-V_dist) * s_NMDA_pathX_dist / (1 + Mg_con * exp(-Alpha_NMDA*(V_dist/mV+Gamma_NMDA)) / Beta_NMDA) * w_NMDA_pathX_dist :amp
ds_NMDA_pathX_dist/dt = -s_NMDA_pathX_dist/t_NMDA_decay_pathX_dist :1
# Define what happens when a presynaptic spike arrives at the synapse
synX = b.Synapses(inputX, group,
on_pre='s_AMPA_pathX_dist += 1; s_NMDA_pathX_dist += 1')
synY = b.Synapses(inputY, group,
on_pre='s_AMPA_pathY_prox += 1; s_NMDA_pathY_prox += 1')
# This is the actual connection part
synX.connect(p=0.5) # 50% of the inputs X connect to the group
synY.connect(p=0.5) # 50% of the inputs Y connect to the group
# Set spike and voltage monitors
S = b.SpikeMonitor(group)
voltages = ['V_soma', 'V_trunk', 'V_prox', 'V_dist']
M = b.StateMonitor(group, voltages, record=True)
# Run simulation
net = b.Network(group, ap_reset, synX, synY, S)
b.run(1000*ms)
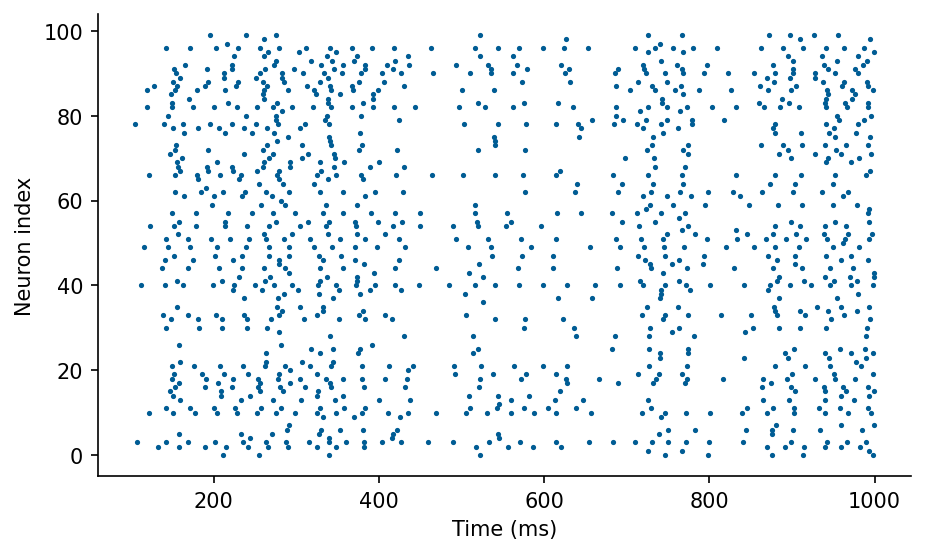
# Plot spiketimes
fig, ax = plt.subplots(figsize=(7, 4))
ax.plot(S.t/ms, S.i, '.')
ax.set_xlabel('Time (ms)')
ax.set_ylabel('Neuron index');
# Plot voltages
fig, ax = plt.subplots(figsize=(7, 4))
ax.plot(M.t/ms, M.V_soma[0]/mV, label='soma', zorder=3)
ax.plot(M.t/ms, M.V_trunk[0]/mV, label='trunk')
ax.plot(M.t/ms, M.V_prox[0]/mV, label='prox')
ax.plot(M.t/ms, M.V_dist[0]/mV, label='dist')
ax.set_xlabel('Time (ms)')
ax.set_ylabel('Voltage (mV)')
ax.legend(loc='best');
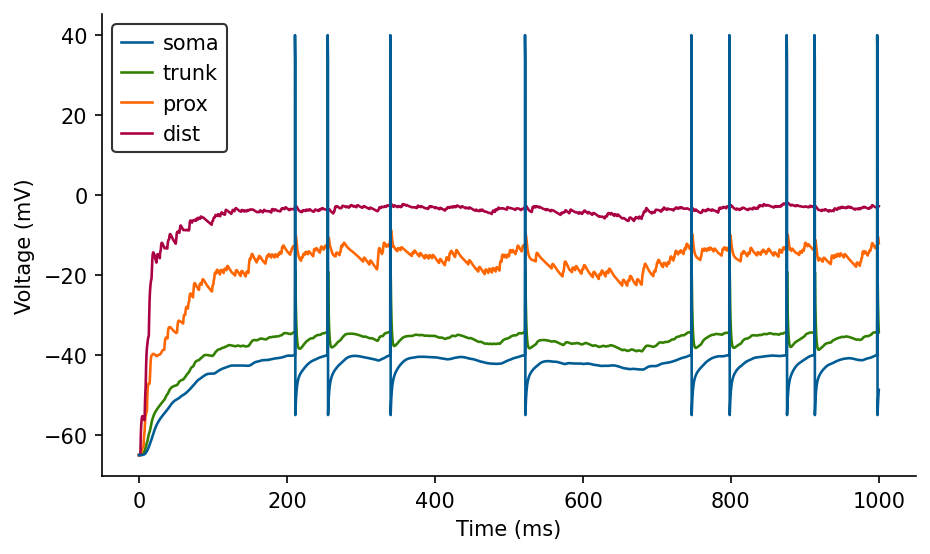
Playing with dSpikes#
b.start_scope() # clear previous run
# add channels to compartments
dist.dspikes('Na', g_rise=3.7*nS, g_fall=2.4*nS)
prox.dspikes('Na', g_rise=9*nS, g_fall=5.7*nS)
trunk.dspikes('Na', g_rise=22*nS, g_fall=14*nS)
model = NeuronModel(graph, cm=1*uF/(cm**2), gl=40*uS/(cm**2),
v_rest=-65*mV, r_axial=150*ohm*cm,
scale_factor=2.8, spine_factor=1.5)
model.config_dspikes('Na', threshold=-35*mV,
duration_rise=1.2*ms, duration_fall=2.4*ms,
offset_fall=0.2*ms, refractory=5*ms,
reversal_rise='E_Na', reversal_fall='E_K')
print(model)
OBJECT
------
<class 'dendrify.neuronmodel.NeuronModel'>
EQUATIONS
---------
dV_soma/dt = (gL_soma * (EL_soma-V_soma) + I_soma) / C_soma :volt
I_soma = I_ext_soma + I_trunk_soma :amp
I_ext_soma :amp
I_trunk_soma = (V_trunk-V_soma) * g_trunk_soma :amp
dV_trunk/dt = (gL_trunk * (EL_trunk-V_trunk) + I_trunk) / C_trunk :volt
I_trunk = I_ext_trunk + I_prox_trunk + I_soma_trunk + I_rise_Na_trunk + I_fall_Na_trunk :amp
I_ext_trunk :amp
I_rise_Na_trunk = g_rise_Na_trunk * (E_rise_Na-V_trunk) :amp
I_fall_Na_trunk = g_fall_Na_trunk * (E_fall_Na-V_trunk) :amp
g_rise_Na_trunk = g_rise_max_Na_trunk * int(t_in_timesteps <= spiketime_Na_trunk + duration_rise_Na_trunk) * gate_Na_trunk :siemens
g_fall_Na_trunk = g_fall_max_Na_trunk * int(t_in_timesteps <= spiketime_Na_trunk + offset_fall_Na_trunk + duration_fall_Na_trunk) * int(t_in_timesteps >= spiketime_Na_trunk + offset_fall_Na_trunk) * gate_Na_trunk :siemens
spiketime_Na_trunk :1
gate_Na_trunk :1
I_soma_trunk = (V_soma-V_trunk) * g_soma_trunk :amp
I_prox_trunk = (V_prox-V_trunk) * g_prox_trunk :amp
dV_prox/dt = (gL_prox * (EL_prox-V_prox) + I_prox) / C_prox :volt
I_prox = I_ext_prox + I_dist_prox + I_trunk_prox + I_rise_Na_prox + I_fall_Na_prox + I_NMDA_pathY_prox + I_AMPA_pathY_prox :amp
I_ext_prox :amp
I_AMPA_pathY_prox = g_AMPA_pathY_prox * (E_AMPA-V_prox) * s_AMPA_pathY_prox * w_AMPA_pathY_prox :amp
ds_AMPA_pathY_prox/dt = -s_AMPA_pathY_prox / t_AMPA_decay_pathY_prox :1
I_NMDA_pathY_prox = g_NMDA_pathY_prox * (E_NMDA-V_prox) * s_NMDA_pathY_prox / (1 + Mg_con * exp(-Alpha_NMDA*(V_prox/mV+Gamma_NMDA)) / Beta_NMDA) * w_NMDA_pathY_prox :amp
ds_NMDA_pathY_prox/dt = -s_NMDA_pathY_prox/t_NMDA_decay_pathY_prox :1
I_rise_Na_prox = g_rise_Na_prox * (E_rise_Na-V_prox) :amp
I_fall_Na_prox = g_fall_Na_prox * (E_fall_Na-V_prox) :amp
g_rise_Na_prox = g_rise_max_Na_prox * int(t_in_timesteps <= spiketime_Na_prox + duration_rise_Na_prox) * gate_Na_prox :siemens
g_fall_Na_prox = g_fall_max_Na_prox * int(t_in_timesteps <= spiketime_Na_prox + offset_fall_Na_prox + duration_fall_Na_prox) * int(t_in_timesteps >= spiketime_Na_prox + offset_fall_Na_prox) * gate_Na_prox :siemens
spiketime_Na_prox :1
gate_Na_prox :1
I_trunk_prox = (V_trunk-V_prox) * g_trunk_prox :amp
I_dist_prox = (V_dist-V_prox) * g_dist_prox :amp
dV_dist/dt = (gL_dist * (EL_dist-V_dist) + I_dist) / C_dist :volt
I_dist = I_ext_dist + I_prox_dist + I_rise_Na_dist + I_fall_Na_dist + I_NMDA_pathX_dist + I_AMPA_pathX_dist :amp
I_ext_dist :amp
I_AMPA_pathX_dist = g_AMPA_pathX_dist * (E_AMPA-V_dist) * s_AMPA_pathX_dist * w_AMPA_pathX_dist :amp
ds_AMPA_pathX_dist/dt = -s_AMPA_pathX_dist / t_AMPA_decay_pathX_dist :1
I_NMDA_pathX_dist = g_NMDA_pathX_dist * (E_NMDA-V_dist) * s_NMDA_pathX_dist / (1 + Mg_con * exp(-Alpha_NMDA*(V_dist/mV+Gamma_NMDA)) / Beta_NMDA) * w_NMDA_pathX_dist :amp
ds_NMDA_pathX_dist/dt = -s_NMDA_pathX_dist/t_NMDA_decay_pathX_dist :1
I_rise_Na_dist = g_rise_Na_dist * (E_rise_Na-V_dist) :amp
I_fall_Na_dist = g_fall_Na_dist * (E_fall_Na-V_dist) :amp
g_rise_Na_dist = g_rise_max_Na_dist * int(t_in_timesteps <= spiketime_Na_dist + duration_rise_Na_dist) * gate_Na_dist :siemens
g_fall_Na_dist = g_fall_max_Na_dist * int(t_in_timesteps <= spiketime_Na_dist + offset_fall_Na_dist + duration_fall_Na_dist) * int(t_in_timesteps >= spiketime_Na_dist + offset_fall_Na_dist) * gate_Na_dist :siemens
spiketime_Na_dist :1
gate_Na_dist :1
I_prox_dist = (V_prox-V_dist) * g_prox_dist :amp
PARAMETERS
----------
{'Alpha_NMDA': 0.062,
'Beta_NMDA': 3.57,
'C_dist': 6.59734457 * pfarad,
'C_prox': 13.19468915 * pfarad,
'C_soma': 82.46680716 * pfarad,
'C_trunk': 32.98672286 * pfarad,
'EL_dist': -65. * mvolt,
'EL_prox': -65. * mvolt,
'EL_soma': -65. * mvolt,
'EL_trunk': -65. * mvolt,
'E_AMPA': 0. * volt,
'E_Ca': 136. * mvolt,
'E_GABA': -80. * mvolt,
'E_K': -89. * mvolt,
'E_NMDA': 0. * volt,
'E_Na': 70. * mvolt,
'E_fall_Na': -89. * mvolt,
'E_rise_Na': 70. * mvolt,
'Gamma_NMDA': 0,
'Mg_con': 1.0,
'Vth_Na_dist': -35. * mvolt,
'Vth_Na_prox': -35. * mvolt,
'Vth_Na_trunk': -35. * mvolt,
'duration_fall_Na_dist': 24,
'duration_fall_Na_prox': 24,
'duration_fall_Na_trunk': 24,
'duration_rise_Na_dist': 12,
'duration_rise_Na_prox': 12,
'duration_rise_Na_trunk': 12,
'gL_dist': 263.8937829 * psiemens,
'gL_prox': 0.52778757 * nsiemens,
'gL_soma': 3.29867229 * nsiemens,
'gL_trunk': 1.31946891 * nsiemens,
'g_AMPA_pathX_dist': 1. * nsiemens,
'g_AMPA_pathY_prox': 1. * nsiemens,
'g_NMDA_pathX_dist': 1. * nsiemens,
'g_NMDA_pathY_prox': 1. * nsiemens,
'g_dist_prox': 2. * nsiemens,
'g_fall_max_Na_dist': 2.4 * nsiemens,
'g_fall_max_Na_prox': 5.7 * nsiemens,
'g_fall_max_Na_trunk': 14. * nsiemens,
'g_prox_dist': 2. * nsiemens,
'g_prox_trunk': 6. * nsiemens,
'g_rise_max_Na_dist': 3.7 * nsiemens,
'g_rise_max_Na_prox': 9. * nsiemens,
'g_rise_max_Na_trunk': 22. * nsiemens,
'g_soma_trunk': 15. * nsiemens,
'g_trunk_prox': 6. * nsiemens,
'g_trunk_soma': 15. * nsiemens,
'offset_fall_Na_dist': 2,
'offset_fall_Na_prox': 2,
'offset_fall_Na_trunk': 2,
'refractory_Na_dist': 50,
'refractory_Na_prox': 50,
'refractory_Na_trunk': 50,
't_AMPA_decay_pathX_dist': 2. * msecond,
't_AMPA_decay_pathY_prox': 2. * msecond,
't_NMDA_decay_pathX_dist': 60. * msecond,
't_NMDA_decay_pathY_prox': 60. * msecond,
'w_AMPA_pathX_dist': 1.0,
'w_AMPA_pathY_prox': 1.0,
'w_NMDA_pathX_dist': 1.0,
'w_NMDA_pathY_prox': 1.0}
EVENTS
------
['spike_Na_trunk', 'spike_Na_prox', 'spike_Na_dist']
EVENT CONDITIONS
----------------
{'spike_Na_dist': 'V_dist >= Vth_Na_dist and t_in_timesteps >= spiketime_Na_dist + refractory_Na_dist * gate_Na_dist',
'spike_Na_prox': 'V_prox >= Vth_Na_prox and t_in_timesteps >= spiketime_Na_prox + refractory_Na_prox * gate_Na_prox',
'spike_Na_trunk': 'V_trunk >= Vth_Na_trunk and t_in_timesteps >= spiketime_Na_trunk + refractory_Na_trunk * '
'gate_Na_trunk'}
# Make a new neurongroup
neuron, ap_reset = model.make_neurongroup(1, method='euler', threshold='V_soma > -40*mV',
reset='V_soma = 40*mV',
second_reset= 'V_soma=-55*mV',
spike_width = 0.8*ms,
refractory=4*ms)
vars = ['V_soma', 'V_trunk', 'V_prox', 'V_dist']
M = b.StateMonitor(neuron, vars, record=True)
net = b.Network(neuron, ap_reset, M)
net.run(10*ms)
neuron.I_ext_soma = 150*pA
net.run(100*ms)
neuron.I_ext_soma = 0*pA
net.run(60*ms)
# @title Plot voltages
fig, ax = plt.subplots(figsize=(7, 4))
ax.plot(M.t/ms, M.V_soma[0]/mV, label='soma', zorder=3)
ax.plot(M.t/ms, M.V_trunk[0]/mV, label='trunk')
ax.plot(M.t/ms, M.V_prox[0]/mV, label='prox')
ax.plot(M.t/ms, M.V_dist[0]/mV, label='dist')
ax.set_xlabel('Time (ms)')
ax.set_ylabel('Voltage (mV)')
ax.legend(loc='best');
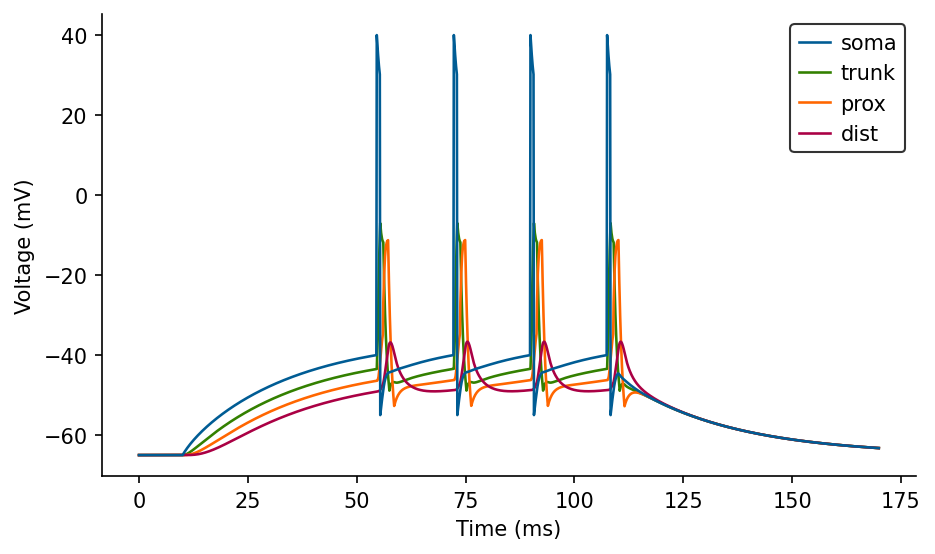
Now these are some actual backpropagating dendritic spikes with sodium-like characteristics.
# Zoom in
fig, ax = plt.subplots(figsize=(7, 4))
ax.plot(M.t/ms, M.V_soma[0]/mV, label='soma')
ax.plot(M.t/ms, M.V_trunk[0]/mV, label='trunk')
ax.plot(M.t/ms, M.V_prox[0]/mV, label='prox')
ax.plot(M.t/ms, M.V_dist[0]/mV, label='dist')
ax.set_xlabel('Time (ms)')
ax.set_ylabel('Voltage (mV)')
ax.set_xlim(left=50, right=100)
ax.legend(loc='best');
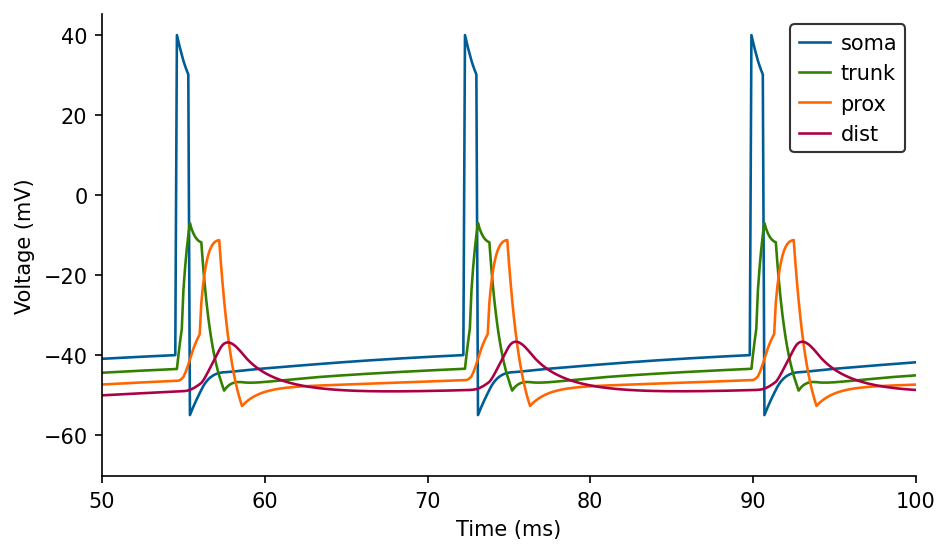
Adding some noise#
b.start_scope() # clear previous run
# add noise
a = 2
soma.noise(mean=a*25*pA, sigma=25*pA, tau=1*ms)
trunk.noise(mean=a*20*pA, sigma=20*pA, tau=1*ms)
prox.noise(mean=a*15*pA, sigma=15*pA, tau=1*ms)
dist.noise(mean=a*6*pA, sigma=10*pA, tau=1*ms)
# merge compartments into a neuron model and set its basic properties
edges = [(soma, trunk, 15*nS), (trunk, prox, 8*nS), (prox, dist, 3*nS)]
model = NeuronModel(edges, cm=1*uF/(cm**2), gl=40*uS/(cm**2),
v_rest=-65*mV, r_axial=150*ohm*cm,
scale_factor=2.8, spine_factor=1.5)
model.config_dspikes('Na', threshold=-35*mV,
duration_rise=1.2*ms, duration_fall=2.4*ms,
offset_fall=0.2*ms, refractory=5*ms,
reversal_rise='E_Na', reversal_fall='E_K')
# make a neuron group
neuron, ap_reset = model.make_neurongroup(1, method='euler', threshold='V_soma > -40*mV',
reset='V_soma = 40*mV',
second_reset= 'V_soma=-55*mV',
spike_width = 0.8*ms,
refractory=4*ms)
# record voltages
vars = ['V_soma', 'V_trunk', 'V_prox', 'V_dist']
M = b.StateMonitor(neuron, vars, record=True)
print(model)
OBJECT
------
<class 'dendrify.neuronmodel.NeuronModel'>
EQUATIONS
---------
dV_soma/dt = (gL_soma * (EL_soma-V_soma) + I_soma) / C_soma :volt
I_soma = I_ext_soma + I_trunk_soma + I_noise_soma :amp
I_ext_soma :amp
dI_noise_soma/dt = (mean_noise_soma-I_noise_soma) / tau_noise_soma + sigma_noise_soma * (sqrt(2/(tau_noise_soma*dt)) * randn()) :amp
I_trunk_soma = (V_trunk-V_soma) * g_trunk_soma :amp
dV_trunk/dt = (gL_trunk * (EL_trunk-V_trunk) + I_trunk) / C_trunk :volt
I_trunk = I_ext_trunk + I_prox_trunk + I_soma_trunk + I_noise_trunk + I_rise_Na_trunk + I_fall_Na_trunk :amp
I_ext_trunk :amp
I_rise_Na_trunk = g_rise_Na_trunk * (E_rise_Na-V_trunk) :amp
I_fall_Na_trunk = g_fall_Na_trunk * (E_fall_Na-V_trunk) :amp
g_rise_Na_trunk = g_rise_max_Na_trunk * int(t_in_timesteps <= spiketime_Na_trunk + duration_rise_Na_trunk) * gate_Na_trunk :siemens
g_fall_Na_trunk = g_fall_max_Na_trunk * int(t_in_timesteps <= spiketime_Na_trunk + offset_fall_Na_trunk + duration_fall_Na_trunk) * int(t_in_timesteps >= spiketime_Na_trunk + offset_fall_Na_trunk) * gate_Na_trunk :siemens
spiketime_Na_trunk :1
gate_Na_trunk :1
dI_noise_trunk/dt = (mean_noise_trunk-I_noise_trunk) / tau_noise_trunk + sigma_noise_trunk * (sqrt(2/(tau_noise_trunk*dt)) * randn()) :amp
I_soma_trunk = (V_soma-V_trunk) * g_soma_trunk :amp
I_prox_trunk = (V_prox-V_trunk) * g_prox_trunk :amp
dV_prox/dt = (gL_prox * (EL_prox-V_prox) + I_prox) / C_prox :volt
I_prox = I_ext_prox + I_dist_prox + I_trunk_prox + I_noise_prox + I_rise_Na_prox + I_fall_Na_prox + I_NMDA_pathY_prox + I_AMPA_pathY_prox :amp
I_ext_prox :amp
I_AMPA_pathY_prox = g_AMPA_pathY_prox * (E_AMPA-V_prox) * s_AMPA_pathY_prox * w_AMPA_pathY_prox :amp
ds_AMPA_pathY_prox/dt = -s_AMPA_pathY_prox / t_AMPA_decay_pathY_prox :1
I_NMDA_pathY_prox = g_NMDA_pathY_prox * (E_NMDA-V_prox) * s_NMDA_pathY_prox / (1 + Mg_con * exp(-Alpha_NMDA*(V_prox/mV+Gamma_NMDA)) / Beta_NMDA) * w_NMDA_pathY_prox :amp
ds_NMDA_pathY_prox/dt = -s_NMDA_pathY_prox/t_NMDA_decay_pathY_prox :1
I_rise_Na_prox = g_rise_Na_prox * (E_rise_Na-V_prox) :amp
I_fall_Na_prox = g_fall_Na_prox * (E_fall_Na-V_prox) :amp
g_rise_Na_prox = g_rise_max_Na_prox * int(t_in_timesteps <= spiketime_Na_prox + duration_rise_Na_prox) * gate_Na_prox :siemens
g_fall_Na_prox = g_fall_max_Na_prox * int(t_in_timesteps <= spiketime_Na_prox + offset_fall_Na_prox + duration_fall_Na_prox) * int(t_in_timesteps >= spiketime_Na_prox + offset_fall_Na_prox) * gate_Na_prox :siemens
spiketime_Na_prox :1
gate_Na_prox :1
dI_noise_prox/dt = (mean_noise_prox-I_noise_prox) / tau_noise_prox + sigma_noise_prox * (sqrt(2/(tau_noise_prox*dt)) * randn()) :amp
I_trunk_prox = (V_trunk-V_prox) * g_trunk_prox :amp
I_dist_prox = (V_dist-V_prox) * g_dist_prox :amp
dV_dist/dt = (gL_dist * (EL_dist-V_dist) + I_dist) / C_dist :volt
I_dist = I_ext_dist + I_prox_dist + I_noise_dist + I_rise_Na_dist + I_fall_Na_dist + I_NMDA_pathX_dist + I_AMPA_pathX_dist :amp
I_ext_dist :amp
I_AMPA_pathX_dist = g_AMPA_pathX_dist * (E_AMPA-V_dist) * s_AMPA_pathX_dist * w_AMPA_pathX_dist :amp
ds_AMPA_pathX_dist/dt = -s_AMPA_pathX_dist / t_AMPA_decay_pathX_dist :1
I_NMDA_pathX_dist = g_NMDA_pathX_dist * (E_NMDA-V_dist) * s_NMDA_pathX_dist / (1 + Mg_con * exp(-Alpha_NMDA*(V_dist/mV+Gamma_NMDA)) / Beta_NMDA) * w_NMDA_pathX_dist :amp
ds_NMDA_pathX_dist/dt = -s_NMDA_pathX_dist/t_NMDA_decay_pathX_dist :1
I_rise_Na_dist = g_rise_Na_dist * (E_rise_Na-V_dist) :amp
I_fall_Na_dist = g_fall_Na_dist * (E_fall_Na-V_dist) :amp
g_rise_Na_dist = g_rise_max_Na_dist * int(t_in_timesteps <= spiketime_Na_dist + duration_rise_Na_dist) * gate_Na_dist :siemens
g_fall_Na_dist = g_fall_max_Na_dist * int(t_in_timesteps <= spiketime_Na_dist + offset_fall_Na_dist + duration_fall_Na_dist) * int(t_in_timesteps >= spiketime_Na_dist + offset_fall_Na_dist) * gate_Na_dist :siemens
spiketime_Na_dist :1
gate_Na_dist :1
dI_noise_dist/dt = (mean_noise_dist-I_noise_dist) / tau_noise_dist + sigma_noise_dist * (sqrt(2/(tau_noise_dist*dt)) * randn()) :amp
I_prox_dist = (V_prox-V_dist) * g_prox_dist :amp
PARAMETERS
----------
{'Alpha_NMDA': 0.062,
'Beta_NMDA': 3.57,
'C_dist': 6.59734457 * pfarad,
'C_prox': 13.19468915 * pfarad,
'C_soma': 82.46680716 * pfarad,
'C_trunk': 32.98672286 * pfarad,
'EL_dist': -65. * mvolt,
'EL_prox': -65. * mvolt,
'EL_soma': -65. * mvolt,
'EL_trunk': -65. * mvolt,
'E_AMPA': 0. * volt,
'E_Ca': 136. * mvolt,
'E_GABA': -80. * mvolt,
'E_K': -89. * mvolt,
'E_NMDA': 0. * volt,
'E_Na': 70. * mvolt,
'E_fall_Na': -89. * mvolt,
'E_rise_Na': 70. * mvolt,
'Gamma_NMDA': 0,
'Mg_con': 1.0,
'Vth_Na_dist': -35. * mvolt,
'Vth_Na_prox': -35. * mvolt,
'Vth_Na_trunk': -35. * mvolt,
'duration_fall_Na_dist': 24,
'duration_fall_Na_prox': 24,
'duration_fall_Na_trunk': 24,
'duration_rise_Na_dist': 12,
'duration_rise_Na_prox': 12,
'duration_rise_Na_trunk': 12,
'gL_dist': 263.8937829 * psiemens,
'gL_prox': 0.52778757 * nsiemens,
'gL_soma': 3.29867229 * nsiemens,
'gL_trunk': 1.31946891 * nsiemens,
'g_AMPA_pathX_dist': 1. * nsiemens,
'g_AMPA_pathY_prox': 1. * nsiemens,
'g_NMDA_pathX_dist': 1. * nsiemens,
'g_NMDA_pathY_prox': 1. * nsiemens,
'g_dist_prox': 3. * nsiemens,
'g_fall_max_Na_dist': 2.4 * nsiemens,
'g_fall_max_Na_prox': 5.7 * nsiemens,
'g_fall_max_Na_trunk': 14. * nsiemens,
'g_prox_dist': 3. * nsiemens,
'g_prox_trunk': 8. * nsiemens,
'g_rise_max_Na_dist': 3.7 * nsiemens,
'g_rise_max_Na_prox': 9. * nsiemens,
'g_rise_max_Na_trunk': 22. * nsiemens,
'g_soma_trunk': 15. * nsiemens,
'g_trunk_prox': 8. * nsiemens,
'g_trunk_soma': 15. * nsiemens,
'mean_noise_dist': 12. * pamp,
'mean_noise_prox': 30. * pamp,
'mean_noise_soma': 50. * pamp,
'mean_noise_trunk': 40. * pamp,
'offset_fall_Na_dist': 2,
'offset_fall_Na_prox': 2,
'offset_fall_Na_trunk': 2,
'refractory_Na_dist': 50,
'refractory_Na_prox': 50,
'refractory_Na_trunk': 50,
'sigma_noise_dist': 10. * pamp,
'sigma_noise_prox': 15. * pamp,
'sigma_noise_soma': 25. * pamp,
'sigma_noise_trunk': 20. * pamp,
't_AMPA_decay_pathX_dist': 2. * msecond,
't_AMPA_decay_pathY_prox': 2. * msecond,
't_NMDA_decay_pathX_dist': 60. * msecond,
't_NMDA_decay_pathY_prox': 60. * msecond,
'tau_noise_dist': 1. * msecond,
'tau_noise_prox': 1. * msecond,
'tau_noise_soma': 1. * msecond,
'tau_noise_trunk': 1. * msecond,
'w_AMPA_pathX_dist': 1.0,
'w_AMPA_pathY_prox': 1.0,
'w_NMDA_pathX_dist': 1.0,
'w_NMDA_pathY_prox': 1.0}
EVENTS
------
['spike_Na_trunk', 'spike_Na_prox', 'spike_Na_dist']
EVENT CONDITIONS
----------------
{'spike_Na_dist': 'V_dist >= Vth_Na_dist and t_in_timesteps >= spiketime_Na_dist + refractory_Na_dist * gate_Na_dist',
'spike_Na_prox': 'V_prox >= Vth_Na_prox and t_in_timesteps >= spiketime_Na_prox + refractory_Na_prox * gate_Na_prox',
'spike_Na_trunk': 'V_trunk >= Vth_Na_trunk and t_in_timesteps >= spiketime_Na_trunk + refractory_Na_trunk * '
'gate_Na_trunk'}
# run simulation
net = b.Network(neuron, ap_reset, M)
net.run(500*ms)
# @title Plot voltages
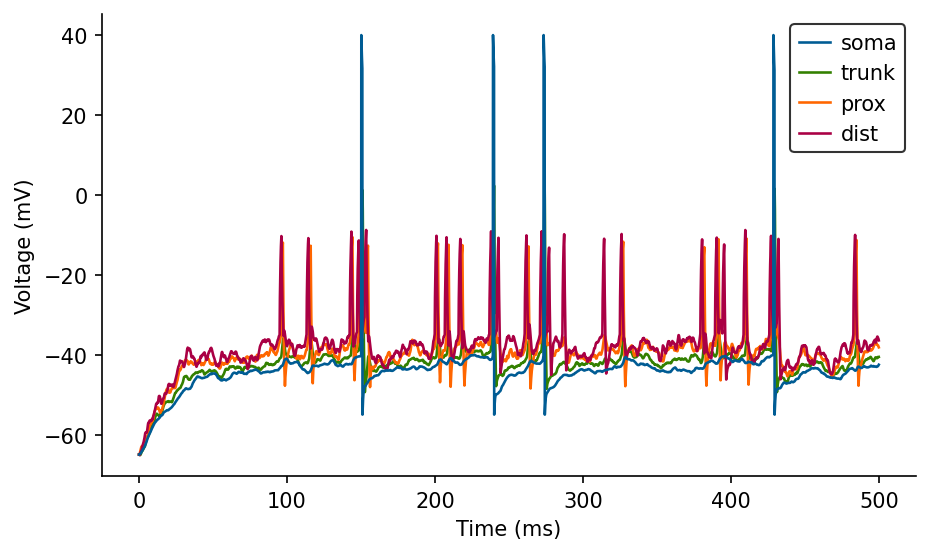
fig, ax = plt.subplots(figsize=(7, 4))
ax.plot(M.t/ms, M.V_soma[0]/mV, label='soma', zorder=3)
ax.plot(M.t/ms, M.V_trunk[0]/mV, label='trunk')
ax.plot(M.t/ms, M.V_prox[0]/mV, label='prox')
ax.plot(M.t/ms, M.V_dist[0]/mV, label='dist')
ax.set_xlabel('Time (ms)')
ax.set_ylabel('Voltage (mV)')
ax.legend(loc='best');
Point neurons#
from dendrify import PointNeuronModel
b.start_scope()
# create a point-neuron model and add some Poisson input
point_model = PointNeuronModel(model='leakyIF', v_rest=-60*mV,
cm_abs=200*pF, gl_abs=10*nS)
point_model.synapse('AMPA', tag='x', g=1*nS, t_decay=2*ms)
point_neuron = point_model.make_neurongroup(1, method='euler') # no spiking
Input = b.PoissonGroup(10, rates=100*Hz)
S = b.Synapses(Input, point_neuron, on_pre='s_AMPA_x += 1')
S.connect(p=1)
# monitor
M2 = b.StateMonitor(point_neuron, 'V', record=True)
# simulation
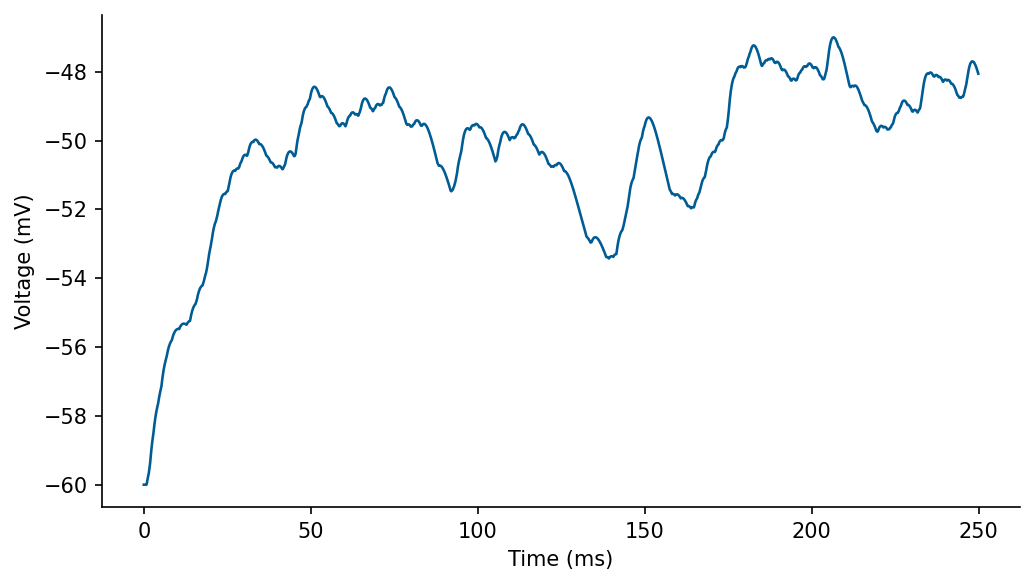
b.run(250*ms)
# plot
fig, ax = plt.subplots(1, 1, figsize=[7, 4])
ax.plot(M2.t/ms, M2.V[0]/mV)
ax.set_xlabel('Time (ms)')
ax.set_ylabel('Voltage (mV)')
fig.tight_layout();